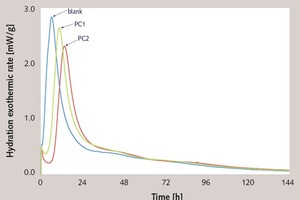
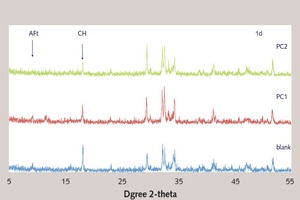
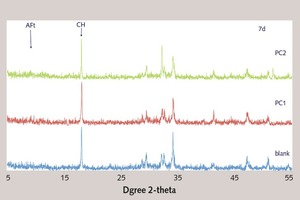
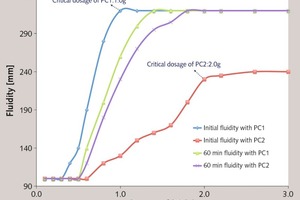
Effect of the ester group in side chain of polycarboxylate superplasticizer on the cement hydration process
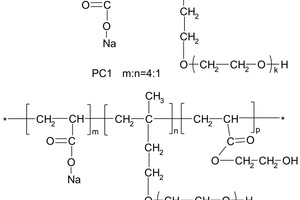
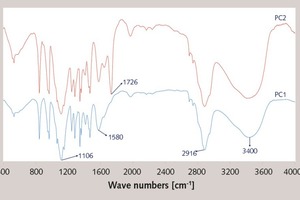
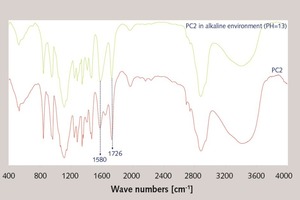
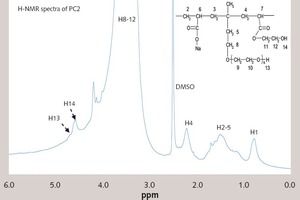
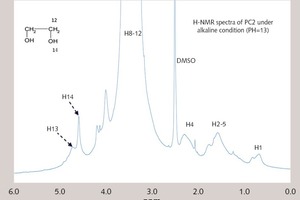
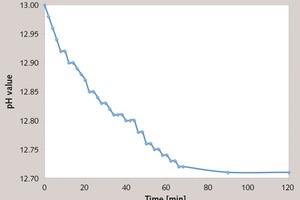
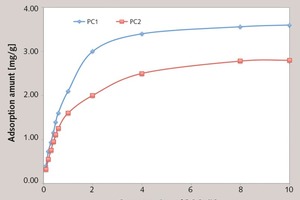
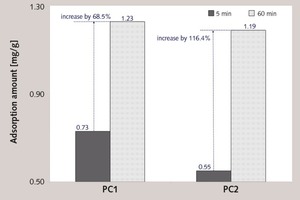
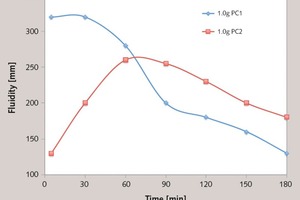
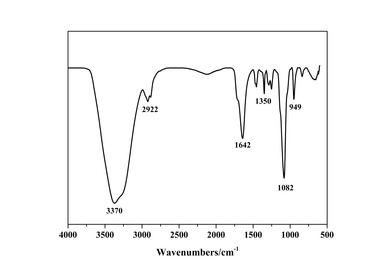
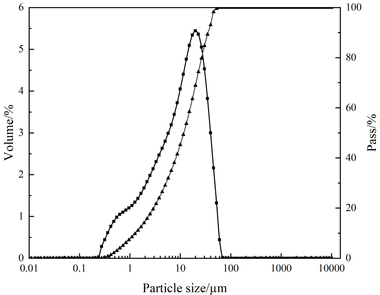
In this paper, the effect of the presence of the ester group in side chain of polycarboxylate superplasticizer (PC) on the cement hydration process was discussed. PC without the ester group was synthesized with acrylic acid (AA) and isoprenyl polyoxyethylene (IPEG), and PC2 with the ester group was synthesized with acrylic acid (AA), hydroxyethyl acrylate (HEA) and IPEG. The chemical structure of copolymer was investigated by FTIR and GPC. The stability of the ester group in side chain was tested by FTIR, pH value and 1H-NMR, and the adsorption amount was measured by TOC. Then the hydration process of cement paste with additional copolymer was examined by hydration heat and XRD. The results show that: under the alkaline environment in the cement hydration process, the ester group in side chain can be decomposed into the carboxyl group and glycol. And the presence of the ester group in side chain can weaken the initial adsorbing ability and obviously enhance the later adsorbing ability, because of the increase of the carboxyl group originating from the decomposition of the ester group. PC with the ester group has less initial dispersion, and the decomposition of the ester group can enhance its later dispersion, which is the main reason for the fluidity increase of cement paste over time. It is suggested that PC containing the ester group in side chain can be used to delay the cement hydration and improve the fluidity loss of fresh cement paste and concrete.
1 Introduction
In recent years, the cement-based materials have been significantly improved by high performance superplasticizer [1-4], which is shown as the high fluidity of the fresh concrete [5-7] and the low porosity of hardened concrete by using less water [8-9]. Polycarboxylate superplasticizer (PC), which consists of one main chain and many side chains, is well known as the most efficient superplasticizer [10-12]. Steric hindrance, provided by the long side chain such as polyethylene oxide (PEO), is the main reason for its high efficient dispersion [13-14]. The length of the long side...