Slags from iron and steel plants and their use
in the building materials industry
The authors of the paper “Characterization of the microstructure and mineral phases of slags from German iron and steel plants” [1] investigated the mineral composition of metallurgical slags and their use in the building materials industry, also against the backdrop of an alkali-silica-reaction in the concrete. The FEhS-Institut für Baustoff-Forschung (Institute for Building Materials Research of the Metallurgical Slags Research Association) has been dealing with this topic for a long time and was interested in getting some more answers as regards some of the facts. Dr.-Ing. Peter Drissen from the FEhS-Institut für Baustoff-Forschung, Duisburg/Germany, had a corresponding conversation with Dr. Zhao from the University of Technology in Wuhan/China.
Qinglin Zhao: The high level of utilization means that a lot of positive experience has been gathered, in particular in Germany, as regards the use of these materials. For this reason I would like to find out, within the framework of a research stay in Weimar, what are the qualities for which fields of application. I think that this experience could be very useful for us in China. In the meantime there are similar approaches in China to make use of industrial by-products. However, these approaches have not progressed as far as here in Germany. On the other hand, this gives us the opportunity to pursue new approaches, which would not be possible in Germany without further difficulties. For instance, first experience has been gathered in China as regards the use of ground LD slag in concrete or bricks. Therefore, I am also interested in the behaviour with regard to a potential alkali-silica-reaction.
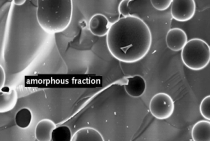
Qinglin Zhao: In this case silica/silicic acid was only mathematically determined from the element Si based on the SEM/EDX investigations. As an amorphous constituent this is a positive assessment for the ASR since Si as very fine particles may have a buffering effect on the alkalis.
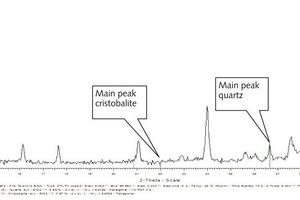
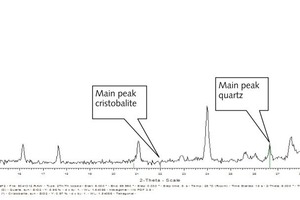
Qinglin Zhao: In the investigated lumpy blastfurnace slag 1.3 % of quartz were detected as crystalline constituent. Christobalite could not be detected. Figure 1 shows the diagram of the X-ray crystal analysis of a lumpy blastfurnace slag as an example. It clearly shows the main peak of quartz, even if the amount of quartz is very small. The main peak of christobalite does not exist.
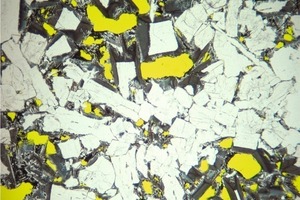
Qinglin Zhao: The connection between the vitreous portion and the cooling rate is generally known. Therefore, as I see it, lumpy blastfurnace slag may also contain amorphous portions. The crystalline portions of the slag mentioned in the paper were determined by means of a quantitative X-ray crystal analysis, which is very exact. The analytical balance, as opposed to the chemical analysis, i. e. the proximate composition, was then assigned to the “X-ray amorphous” or vitreous portion, respectively. It resulted in an amorphous portion of approximately 24 % for the lumpy blastfurnace slag investigated in this case. Figure 2 shows the microstructure of blastfurnace slag under the microscope. It can clearly be seen that the blastfurnace slag has already been well crystallized.
The detection of sulphates and gypsum, respectively, in places in your published images also argue in favour of older, deposited samples. Since hydration and carbonatization products have a great number of different mineral phases, as a rule, it is not possible or only very difficult to identify them by means of X-ray photography. Furthermore, the thin-walled structures are mostly smaller than the diameter of the electron ray used for the SEM/EDX analyses. Due to the inexact analytical chemistry these ranges cannot be assigned to any mineral phase, particularly as the portions of water of hydration and CO2, respectively, are not identified either.
Qinglin Zhao: Referring to the properties of old, deposited samples it is certainly important and helpful for the interpretation of the test results. We will take that into account for future explanations.
Qinglin Zhao: The amorphous portion of the investigated samples was also determined with internal standard via XRD. I conclude from your explanations that it certainly would have been clearer to list the portions specified in the tables separately as amorphous silicic acid and calcium dioxide as constituent of a mixed phase.
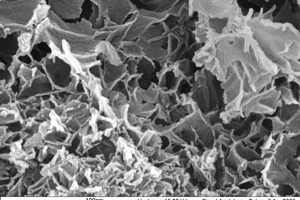
Qinglin Zhao: Yes, it is known that wüstite and iron may exist as constituents in LD or electric furnace slags. The presence of heamatite in these slags is really not very typical. However, the formation of heamatite is possible during cooling and also during storage in the open air in the presence of humidity. This is shown very clearly in Figure 4 in [1]. However, the heamatite content is clearly below 3 %.

![3 SEM-microfabric of GBFS [1]](https://www.zkg.de/imgs/tok_c2f4cd15064610448fc1df6991a1ca30/w300_h200_x310_y326_101520492_5744a53cde.jpg)
![5 SEM-image of LD slag [1]](https://www.zkg.de/imgs/tok_4b9447c8962d9af0b8062dc09e60abef/w300_h200_x316_y316_101520479_cd6f834e64.jpg)