Optimization of the activation process for natural anhydrite
The aim of the present work is to provide the conditions for the activation of natural anhydrite in order to use it as a binder suitable for applications in the building industry. This natural anhydrite appears mixed with the calcium dihydrate in mining operations. Chemical activation of anhydrite is set out exclusively by dosage of activators of different nature: ground granulated blast furnace slag, ground glass residue, gypsum, potassium sulfate, iron (II) sulfate, calcium hydroxide and cement. The degree of hydration was determined by the mass loss method according to UNE-EN 101042. A degree of hydration of 57.56% has been achieved with small doses of cement (3%) and potassium sulfate (1%). Other dosages also offered the minimum chemical requirements established in the standards specifications. Preliminary results reported compressive strengths up to 27 MPa.
1 Introduction
It is a common practice to use industrial and mining wastes as well as by-products for manufacturing binders with a reduced environmental footprint [1-3].
In the gypsum industry, different by-products have been used with the aim of reducing the industrial wastes generated, such as phosphogypsum [4-7], fluogypsum [8-10], and flue gas desulfurisation (FGD) [11]. There are mining operations dedicated to the plaster industry in which, due to its geological characteristics, abundant natural anhydrite appears, which is discarded and set aside because it is not suitable to manufacture construction plaster.
Natural gypsum mining resources in Alicante/Spain belong to the Triassic geological period [12]. In these gypsum formations there are large amounts of natural anhydrite, CaSO4 [13], that coexists with gypsum dihydrate, CaSO4·2H2O, and other minor impurities such as dolomite, limestone and clay.
This work is focused on the reuse of this natural anhydrite as a construction material which is actually highly present in some mining operations in Alicante. Its use as a binder has been considered, taking advantage of its hydration capacity, by activating it with different chemical species and without thermal treatment. Therefore, a process of valorization of the anhydrite residue with low energy consumption is proposed. In comparison to other conglomerates already on the market, this paper provides a material that is sustainable from the industrial and environmental point of view.
The hydration reaction of the anhydrite is:
CaSO4 + 2H2O CaSO4·2H2O
This hydration reaction is very slow, due to the low solubility of the anhydrite which is 2.0 g/l at 20° C [14]. The anhydrite would need to be activated to set in a reasonable time for its commercial application.
Activation of anhydrite can be carried out in several ways. One the procedures includes physical activation by grinding the anhydrite [15]. The common values found for particle sizes were below 90 μm and a Blaine’s specific surface between 4000 and 5000 cm2/g [15-17].
On the other hand, it is possible to work with the chemical activation using mixtures of potassium sulfate and calcium hydroxide as activating products [16, 18-20]. The best reported result offered a degree of hydration of 47.8%, and the dosage was 2% K2SO4 and 0.3% Ca(OH)2 [18]. These authors used this last mixture as a base of work to improve the degree of hydration incorporating different chemical reactants in the composition. These reactants included FeSO4·7H2O, ZnSO4·H2O and KAl(SO4)2 ·12H2O, with dosages of 1%, and a degree of hydration of 79%, 76% and 72% was obtained, respectively, but those mixes offered acidic pH. With the addition of 0.3% of CuSO4, 0.3% of Na2SO4, and 3% of (NH4)2SO4, degrees of hydration of 48%, 100%, 67% were obtained, respectively. These authors [18] detected a great influence of the pH of the mixture on the final properties of the different mixtures, observing that high strengths were achieved in an acidic medium (pH 4.5-7).
Other authors [21] have studied the microstructure development and its relation to the flexural strength. For all their formulations, a water/anhydrite ratio of 0.21 was used, 5.7·10-3 mols of activator were added per 100 g of anhydrite, and mixing water included 1% of plasticizer with respect to the quantity of anhydrite. The following degrees of hydration were obtained for each type of activator: 57% for ZnSO4, 54% for (NH4)2SO4, 44% for Na2SO4, 34% for MgSO4 and 27% for K2SO4.
In addition, another work studied the reactivity of a thermal anhydrite obtained by calcination of FGD gypsum [16]. Values of the degree of hydration around 50% were obtained with additions of K2SO4 between 0.5% and 3.3%, without the addition of lime. The authors proposed that the hydration mechanisms are associated with the formation and stability of double salts.
Other authors have studied the mechanisms of chemical activation of anhydrite through the addition of blast furnace slag [22] using Ca(OH)2, Na2SO4·10H2O and FeSO4·7H2O as activator. The anhydrite used in these experiments was obtained from the thermal treatment of phosphogypsum at 750° C, and their best result was achieved with an anhydrite/slag ratio of 1.0, offering a combined water value of 5.84%.
Other works studied thermal activation of anhydrite together with alkalis and recycled glass and low furnace dust. Their authors reported degrees of hydration up to 70%-76% [23-24].
Additionally, the hydraulic properties of the natural anhydrite was explored by incorporating pozzolans and cements [17, 19]. The reactivity of natural anhydrite was studied, using K2SO4 (0.4%) and CEM-I (1%) as activators in mortars with superplastizicers, and an adequate setting behavior and excellent strength development was reported [25].
The main objective of the present paper is to evaluate the different activation options of natural anhydrite to achieve the highest degree of hydration without thermal activation with a pH above 7. Therefore, we discard the options of working with substances that are effective in acid environments such as (NH4)2SO4 because they can induce the corrosion of copper, bronze, ferric metals and alloys, which are typically in contact with this type of binders in their applications. CuSO4 has been also discarded because it is highly corrosive for steel, iron and galvanized metals. Other activators such as Na2SO4 and MgSO4 can cause efflorescence. The optimal formulation will serve as a basis for prescribing a sustainable binder, whose main application is intended for self-levelling screeds.
2 Experimental procedure
2.1 Raw materials
Natural anhydrite was supplied by Canteras de Yesos Lillo S.L., from the quarry “Loma Viudes” located in Alicante/Spain. Blast furnace slag was provided by the company LafargeHolcim, and recycled glass (with a particle size below 45 μm) from Camacho Recycling, S.L.
Table 1 shows the chemical composition of the main basic raw materials that has been determined by X-ray fluorescence (XRF).
Fineness of the raw materials has been determined by Blaine’s method according to UNE-EN 196-6: 2010 standard [26]. The natural anhydrite was ground using a roller mill with a dynamic separator, and a specific surface of 6770 cm2/g was obtained. The commercial blast furnace slag offered a specific surface area of 5257 cm2/g and for the glass residue the fineness was 6500 cm2/g.
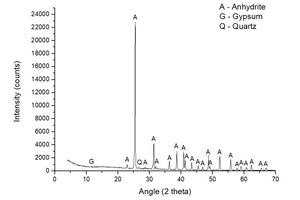
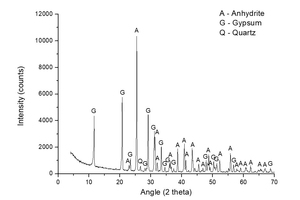
Figure 1 shows the characterization of natural anhydrite made by XRD. Dominant peaks of anhydrite and small peaks of gypsum and quartz are observed.
2.2 Activators
The activators used in this work are potassium sulfate (K2SO4), industrial grade, from Tarazona S.L.; calcium hydroxide Ca(OH)2, from Calcinor; iron (II) sulfate (FeSO4·7H2O) from Aris Industrial S.A., gypsum (CaSO4·2H2O) provided by Mineralis Lucentum S.L. and ordinary Portland cement type I provided by Cemex.
2.3 Methodology
Table 2 shows the nomenclature used for identifying the different materials used in the mixes, and Table 3 shows the different proposed dosages. The proportions of the constituents have been expressed with respect to the total binder mass, i.e., the amount of all the raw materials and activators of a particular mix.
The activators are weighed and mixed with the dry anhydrite, according to the formulation planned in Table 3, with a total weight of 200 g of the materials. They were mixed with the quantity of distilled water established by the standard UNE-13454-2 [27] in order to obtain the standard consistency. The resulting water to binder ratio was 0.45 in all cases, except in the mixtures with slag that was 0.50. The mixing was done by adding the powdered product to the mixing water. Then, the mix stood for 1 minute so that the powder was well saturated with water and, finally, it was stirred for 1 minute. After the mixing, the paste was poured into conical trunk moulds until the paste set. The testing specimens were stored for 24 hours in an atmosphere with a relative humidity higher than 95% and at 20° C. After the setting, the specimens were removed from the moulds and maintained at a relative humidity of 60±5%. The specimens were stored for 7 days under these conditions except for the samples prepared with slag, which were cured for 28 days. To determine the percentage of combined water, the procedure of the UNE-102042 standard was applied [28]. The used instrument was a RADWAG desiccator, model MAC-210. The reached temperature was 200° C in the desiccating scale that guarantees the dehydration of the calcium sulfate dihydrate, according to the following endothermic reaction:
CaSO4 · 2H2O (s) CaSO4 (s) + 2H2O (g)
The degree of hydration has been calculated as the percentage resulting from dividing the mass loss obtained in the dehydration at 200° C and the percentage of total water that would contain a sample consisting of pure anhydrite and assuming that all the anhydrite would have totally reacted, becoming dihydrate, whose water hydration weight would be 20.93%.
In all cases, the degree of hydration has been measured at 7 days, except in the mixtures with slag, which has been measured at 28 days. This is due to the fact that anhydrite mixtures without slag present the same coincident result at 7 and 28 days, so it is assumed that the reactivity of the samples have been completed at 7 days in the proposed conditions.
3 Results and discussion
Table 4 shows the degree of hydration of the mixtures (A-KS-CH) which were made by mixing anhydrite with potassium sulfate and calcium hydroxide.
Sample A-KS-CH-1, with 1% of K2SO4 over the total mass of the mixture, presented a slightly acidic pH (5.96), and it reached a degree of hydration of 26.1%. The effect of adding Ca(OH)2 in the range 0-1%, keeping the amount of K2SO4 constant at 1% (samples A-KS-CH-2 to A-KS-CH-5) increased the pH up to 12.4. As a consequence, the paste result was alkaline and the degree of hydration increased progressively. Sample A-KS-CH-5, with a dosage of 1% K2SO4 and 1% Ca(OH)2, achieved a degree of hydration of 42.91%.
The effect of the addition of K2SO4 from 1% to 4%, keeping Ca(OH)2 constant at 1% (samples A-KS-CH-5 to A-KS-CH-8), improved the degree of hydration for the sample A-KS-CH-6, with 2% of K2SO4 and 1% of Ca(OH)2, offering a value of 45.1%. However, higher values of K2SO4 (3-4%) did not improve the reactivity of the anhydrite and a slight decrease of the combined water falling was observed.
In the series A-KS-CH-9 to A-KS-CH-11, in which the content of calcium hydroxide is increased between 2-4%, and K2SO4 is maintained constant at 2%, a maximum value of hydration degree of 46.2% was registered. The maximum reactivity of all the series was offered by sample A-KS-CH-9, with a content of 2% of Ca(OH)2 and 2% of K2 SO4. A loss of effectiveness was observed if the calcium hydroxide content increased above 2%. These results are close to the degree of hydration achieved by other authors [18], although in the present work a higher content of calcium hydroxide was needed. The difference could be attributed to the particular origin of the anhydrites.
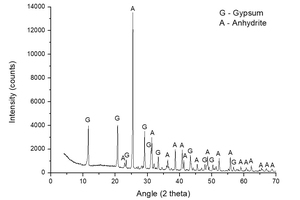
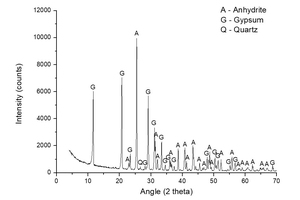
Figure 2 shows a diffractogam of sample A-KS-CH-9, which offered the highest degree of hydration of this series (46.2%), in order to check the products that have been formed in the hydration process. The following mineral species were found: gypsum (CaSO4·2H2O) coming from the hydration reaction, and non-reacted anhydrite (CaSO4). No component with potassium is observed, mainly because the dosage of potassium is very low and therefore not visible in the diffractogram due to the presence of large quantities of highly crystalline products.
In the following series, the influence that blast furnace slag can carry out in the activation of anhydrite was explored. In a first stage, the effect of the activators on the hydration of the slag without anhydrite was studied. For this aim, K2SO4 and calcium hydroxide were mixed with slag and without anhydrite, according to the mixtures (S-KS-CH) proposed in Table 3. The mass loss of the samples at 200° C was measured, as it was done in the whole experiment, but in this case the only purpose of this series was to separate the contribution of the slag hydration products to the mass loss registered for other mixes containing anhydrite and slag.
Table 5 shows the results of mass loss at 200° C of samples prepared with slag, potassium sulfate and calcium hydroxide, and no content of anhydrite. It can be observed that the content of Ca(OH)2 does not modify the degree of hydration, whereas the K2SO4 affects the reactivity of the slag. Sample S-KS-CH-4 reached a mass loss of 4.22%. These data will be used to calculate the contribution of the slag to the mass loss in samples with slag and anhydrite that will be presented in the following series. This has made it possible to clarify the net activity of the anhydrite of that series.
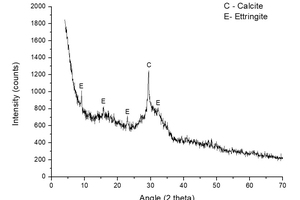
Figure 3 shows the analysis of XRD analysis performed on sample S-KS-CH-4 and it shows the formation of two crystalline phases: calcite, formed by the carbonation of the calcium hydroxide of the sample; and ettringite, formed by the reaction of the sulfates provided by K2SO4 and the calcium aluminates of the slag, in the presence of water [10, 29].
In the following series, the influence of additions of Ca(OH)2 and K2SO4 to mixtures of anhydrite and slag is studied. This proposal is based on the works of other authors [22]. Additionally it should be noted that those authors worked with mixtures which included thermal anhydrite and blast furnace slag. The best results obtained by these authors correspond to the anhydrite/slag ratio of 1.0. This proportion has been adopted in the present research. Table 6 shows the results of the effect of different concentrations of K2SO4 and Ca(OH)2 on the hydration degree of anhydrite/slag pastes. The contribution made by the slag to the mass loss at 200° C is calculated according to data from Table 5. Then, net hydration degree of the anhydrite has been obtained. It can be observed that the highest mass loss is 6.90%, and it corresponds to sample A-S-KS-CH-3. On the other hand, in Table 5, it is observed that the best mass loss value is 4.22%, corresponding to the sample S-KS-CH-4. The highest degree of hydration of the sample A-S-KS-CH-3 mainly comes from the formation of dihydrate from partial hydration of the anhydrite. On the other hand, in sample S-KS-CH-4, hydration would mainly come from the formation of ettringite.
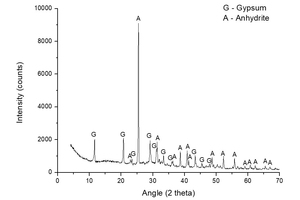
Figure 4 presents the XRD analysis of the sample A-S-KS-CH-3, i.e. the sample that offered the highest mass loss (6.90%), in order to observe the crystalline chemical species that have appeared. The peaks shown in Figure 4 correspond to CaSO4·2H2O (gypsum) and CaSO4 (anhydrite). No ettringite formation is detected, although it may be due to the greater proportion of the abovementioned species. The A-S-KS-CH-3 mixture achieved a mass loss of 6.90%, which is somewhat higher than that obtained by other authors [22] of 5.84%, even taking into account that no heating treatment is applied in the present work.
Table 7 presents the results of the series A-KS-FS-CH, where the effects of the different combinations made with natural anhydrite and other chemical admixtures used as activators are analysed. In this series the influence of K2SO4, FeSO4·7H2O, and Ca(OH)2 is evaluated.
It is noted that the only addition of FeSO4·7H2O does not produce any effect on hydration and does not help to the activation of the anhydrite. If Ca(OH)2 and FeSO4·7H2O are added, the mix reaches alkaline pH but it does not set and no activation is detected. Samples with K2SO4, Ca(OH)2 and FeSO4·7H2O also failed when the pH is higher than 12, unlike the results obtained by other authors [18]. These authors declared a hydration degree of 54% for a mixture of 0.3% Ca(OH)2, 2% K2SO4 and 0.3% FeSO4·7H2O, with a pH of 12.5.
The mixtures of FeSO4·7H2O with K2SO4 and without Ca(OH)2 set and reach very high values of hydration, as is the case with sample A-KS-FS-CH-13, where a maximum hydration degree of 64.3% is achieved. The drawback of this sample arises with the pH, which is 4.50. In the case of other authors [18], their mixture with 3% of FeSO4·7H2O, with a pH of 5, offered a hydration degree of 91%.
To adjust the pH to neutral values, which is required by actual standards, Ca(OH)2 is moderately dosed up to 0.5%. Hydration values up to 55.7% are achieved, such as the one of sample A-KS-FS-CH-20, with additions of 2% K2SO4, 2% FeSO4·7H2O and 0.34% Ca(OH)2. It has been reported a pH 7 with the addition of 1% FeSO4·7H2O, and hydration degree values of 70% [18].
Mixtures whose pH is lower than 7 turns out to be a problem in the construction industry due to the chemical interaction they may have with other construction materials. In that sense, these materials have to comply with the UNE-EN 13454-1 standard, which requires pH > 7 [30].
Due to the sensitivity that the addition of Ca(OH)2 makes in the pH variation, if the addition of Ca(OH)2 reaches values close to 1%, the mix would have a pH higher than 12. The problem, as it is shown in Table 7 (A-KS-FS-CH-9 to A-KS-FS-CH-12), is that it leads to a hydration degree of 0% and no activation of the anhydrite.
Other observed disadvantages are the changes in coloration going from green to red in the sample. This fact could be a possible inconvenience to the use of the product in some building applications. The observed colour changes are in agreement with the information provided by the bibliography [31], in which it is reported that oxidation of Fe (II) to Fe (III) takes place. Despite the fact that other authors have obtained high hydration values [18], it has been decided to not continue with the mixture including FeSO4·7H2O.
Table 8 shows the influence of the dosage of gypsum (DH). In this case the mass loss of the water of crystallization present in the DH has been considered. The quantity has been discounted to the total mass loss to observe the anhydrite activation. It is known that the incorporation of gypsum in calcined gypsum plasters (mainly hemihydrate CaSO4·½H2O) is used as accelerator to produce the rapid hardening of the material. The fact is attributed to the common ion effect and its presence as a crystallization germ, as reported by different authors [32]. For that reason, it was envisaged as a possible activator for the anhydrite. However, the results of Table 8 show that gypsum did not activate the setting of the studied anhydrite, but it behaves as a filler, decreasing the efficiency. However, a quantity of up to 5 % of gypsum could be acceptable, and this proportion could be established as a preventive measure of the limit content of dihydrate in the raw material, which is a mineral that usually appears included in the natural anhydrite.
Table 9 shows the results of the pastes made by adding glass filler to study its possible activating effect. The data in Table 9 show that recycled glass did not contribute to the activation. When the dosage was increased, the water of crystallization decreased, so it can be stated that it acts as an inert admixture. Other authors have obtained high hydration degrees, in the order of 70%, because they applied a thermal activation between 800-900° C over gypsum plus 5% of recycled glass [23].
Table 10 shows the results of the pastes made with cement and potassium sulfate. The degree of hydration was calculated as the net value of mass loss after deduction of the contribution of the cement hydration products at 200° C, which is about 10 % of its mass.
As can be observed in Table 10, the addition of CEM raises the pH up to 12.4 as a consequence of the release of calcium hydroxide in the reaction products of cement hydration.
In the series of samples from A-KS-CEM-1 to A-KS-CEM-8, the potassium sulfate (0.8%) is kept constant and the variation of the cement dosage is analyzed between 1-12%. Increasing hydration degrees are observed as the dosage of cement increases. With the dosage of 9% cement (sample A-KS-CEM-7), a maximum of the net degree of hydration is observed with a value of 57.85%. However, with the following cement addition of 12% (sample A-KS-CEM-8), it is observed that hydration decreases (52.97%).
In the series of samples in Table 10, from A-KS-CEM-9 to A-KS-CEM-14, the cement dosage is kept constant at a value of 3% and the dosage of potassium sulfate is varied from 0.2 to 1.2%. It is observed that the best hydration value corresponds to the sample A-KS-CEM-13, with 3% cement and 1% potassium sulfate, with a net hydration value of 57.56%.
Figure 5 and Figure 6 present the diffractograms of samples A-KS-CEM-7 and A-KS-CEM-13, respectively. In both cases the final composition was similar, presenting the following final products: CaSO4·2H2O (hydrated gypsum), CaSO4 (non-reacted anhydrite) and SiO2 (quartz). No product derived from cement is observed due to its low dosage and the crystalline phases make it difficult to detect minor components. Other authors [19] studied natural anhydrite with high percentages of cement (in the order of 20%) together with pozzolans to obtain hydraulic products similar to cement. They obtained high strengths and hydraulic properties, although this would not be the aim of the present work, in which the scope is to value natural anhydrite using cement as an activator with the minimum dosage of cement.
In summary, Table 11 shows the most relevant dosages in terms of hydration degree. The highest hydration degree was obtained by sample A-KS-FS-CH-13, but the pH was so low that it did not match the standards requirements. For that reason the best performance was given by sample A-KS-CEM-7, consisting of 0.8% K2SO4 and 9% CEM, which offered a hydration degree of 57.8%. However, also sample A-KS-CEM-13 obtained almost the same hydration degree but using a lower proportion of activators (1% K2SO4 and 3% CEM).
In order to test the mechanical performance of the binder, sample A-KS-CEM-13 was selected since it combined an appropriate pH, high hydration degree and low activators content. This dosage was mixed with a powdered superplasticizer additive (0.1% with respect to the total mixture mass of naphthalenesulfonic acid polymer with formaldehyde) to improve its rheological properties, and tests were carried out following the UNE-EN 13454-2 standard [27]. The water/solid ratio was 0.28. The beginning of the setting was detected at 150 min, and it ended after 240 min. The mechanical performance offered 6.0 MPa of flexural strength, and 28 MPa of compressive strength. The workability times and the mechanical strengths are in accordance with the applicable regulation [30]. Other authors [25,33] studied the effect of polycarboxylate superplasticizers in anhydrite mortars for flooring applications, in which the activation was done with K2SO4 and CEM I 42.5R. They obtained results of flexural strength close to 5 MPa [25].
4 Conclusions
It has been demonstrated that natural anhydrite, coming as a residue from gypsum mining operations, can be used as a binder by activating it with discrete amounts of alkaline compounds and without thermal activation. The activating ability of different chemical agents, which are typically used by previous authors, has been studied. It has been concluded that some activating agents such as blast furnace slag, recycled glass powder and natural gypsum do not improve the hydration degree of the natural anhydrite without thermal activation. The use of iron (II) sulfate improves the hydration degree of the anhydrite, but it induces undesired colour changes and yields an acidic environment which is not compatible with other construction materials. On the other hand, for this activator, the setting of the pH to alkaline values is very sensitive to the quantity of CaOH2, and the mix does not set if the pH is above 12. The binder dosage has been optimized by determining the concentrations of cement and potassium sulfate that offer the highest hydration degree. The obtained results show hydration degrees similar to those found by other authors who used thermal anhydrite. The preliminary results of mechanical behaviour show an adequate performance and they are in agreement with the actual required standards for self-levelling screeds.
Acknowledgements
The authors want to acknowledge the technical assistance from Canteras de Yesos Lillo, S.L. and Mineralis Lucentum, S.L.