Principles of fast-setting mortars and concretes
Fast-setting is a requirement for many mortars such as adhesives for natural stones or large format ceramic tiles, tile grouts or self-leveling underlayments. Understanding the mineralogical and technical principles of fast-setting systems is crucial for the design of high-quality mortars and special concretes such as those based on alternative cementitious materials (ACMs). Therefore, this paper provides a twofold overview, a theoretical from the mineralogical perspective, and a practical from the application perspective. In the tetrahedron CaO-(Al,Fe)2O3-SiO2-SO3 a majority of fast-setting systems can be located in a narrow room. Therefore, this tetrahedron is predestinated for a simple graphical overview on the different singular, binary and ternary fast-setting binder systems. From the practical perspective the many additional material properties (e.g., fast drying, shrinkage compensation), which are related to fast-setting mixed binder systems are of great interest. Thus, developing fast-setting mortars or concretes needs a concept to balance the required material properties in a designed formulation.
1 Introduction
Many mortars, such as special adhesives for natural stones or large format ceramic tiles (Sieksmeier 2016 [17]), grouts or self-leveling underlayments (Rego et al. 2021 [15]) are formulated on the basis of fast-setting mixed binder systems. But the development of fast-setting formulations is always a challenging task with respect to the adjustment of workability time and early strength for different ambient temperatures and the high reactivity of the constituents with a sensitivity for ageing (or pre-hydration) during shelf-life (Götz-Neunhoeffer et al. 2009 [5]). Furthermore, there are complex interactions with setting retarders and strength accelerators (Götz-Neunhoeffer and Zurbriggen 2008 [4]), which are needed to control fast-setting in order to gain workability time. The many possibilities to formulate a fast-setting mortar make it difficult for the developer to gain an overview of options from which the right approaches can be selected. Therefore, the goal of this paper is to provide such an overview and possible concepts. For that purpose, the authors combine two perspectives, one from the mineralogy of the binder constituents and the other from the application requirements, which must be met by the corresponding mortar properties.
These two perspectives can be linked by the following key question: How does the mineralogy of fast-setting binder systems relate to mortar properties?
The scope of this study is on fast-setting mixtures based on Portland cement (PC), Calcium aluminate cement (CAC) and calcium sulfoaluminate cement (CSA) with calcium sulfate hemihydrate (CS̅H0.5) or anhydrite (CS̅); for explanation of abbreviations see Table 1.
Together these blended binder systems correspond to the subgroup of “clinkered materials” of Alternative Cementitious Materials (ACMs) as defined by the ACI, the American Concrete Institute (Kurtis et al. 2019 (7)). Because ACMs gain in importance for concrete applications, the know-how of mixed-binder technology for fast-setting mortars becomes increasingly relevant for concrete technology, as well.
Fast-setting formulations cover a broad field of mortar applications such as flooring, tiling, grouting, repairing or waterproofing. The growing demand for fast-setting systems is due to a large number of additional material properties (e.g., Amathieu and Estienne 2003 [1]), which relate to fast-setting mixed binder systems, such as:
early washing of grouts
early over-troweling of second layers
less dependent on application temperature, 5 °C versus 30 °C
early strength buildup
early traffic to shorten waiting times for following construction works
fast drying to reduce drying times to achieve critical residual humidity
hard mortar surface due to hydration prior to drying of the surface layer
dimensional stability by reduced drying shrinkage, or
shrinkage compensation by controlled ettringite formation, resulting in
crack-free appearance (Figure 1)
Figure 2 and Table 2 illustrate and describe a ternary mixed binder system and its pure constituents in their basic properties. Most fast-setting systems, like the ternary mixture in Figure 2 are flash-setting mixtures, which need to be retarded in order to gain a reasonable workability time.
Table 3 is an attempt to provide an overview of the different binder systems, the associated hydrate phase assemblages, the resulting material properties and fields of application. Important to mention is, that this table is not complete, because technical data sheets of commercial products do not declare details regarding the exact binder system. On the other hand, this table can be used for inspiring the development of new products. However, the main goal of this table is to deliver some structure into the vast variety of binder combinations and their material properties.
2 Overview on the mineralogy of fast-setting mortar applications
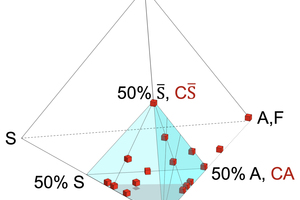
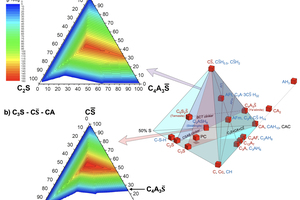
All clinker and hydrate minerals can be described chemically by the following oxides, CaO, Al2O3 (+Fe2O3), SiO2, SO3, and H2O. Disregarding H2O, we can reduce the amount of oxides to the number of four, allowing a tetraplot, a three-dimensional diagram for the visualization of all relevant minerals in the same room (see Figure 3). Because a majority of these minerals plot in the same corner (100% CaO, 50% Al2O3 (+Fe2O3), 50% SiO2, 50% SO3, highlighted in blue) of Figure 3, we can focus in the following to it (see Figures 4 and 5).
3 C-A-S-S̅ tetraplots – an approach for discussing formulation concepts
C-A-S-S̅ tetraplots (Figures 3, 4 and 5) allow a comprehensive illustration of fast-setting systems providing an overview on their mineralogy of clinkers and hydrate phases.
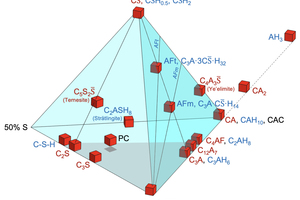
In Figure 4, beside the clinker and hydrate minerals, the approximate mean compositions of Portland cement (PC) and Calcium Aluminate cement (CAC) are plotted. In fact, the main constituents of PC are C3S, C2S, C3A, C4AF, C and CS̅. Therefore, the composition field of PC is near a flat triangle (grey shaded triangle at the bottom of the tetrahedron of Figure 4). Due to the addition of CS̅ during grinding of the Portland cement clinker, the bulk composition of PC is slightly above this grey shaded triangle.
CAC is a term covering the family of Calcium Aluminate cements which strongly vary in the bulk composition along the CA-axis. For reasons of simplicity, the bulk composition of CAC is located at the site of monocalcium aluminate (CA), the main clinker phase of most CACs.
In Figure 5 the fast-setting binder systems are represented as listed in Table 4.
Figure 5 visualizes that the ternary mixed binder systems can be represented by the clinker phases in a narrow space between the two, blue and red shaded triangles. Depending on the exact mixing ratios, ettringite (AFt), monosulfate (AFm), strätlingite (C2ASH8), calcium-aluminate-hydrates (CAH10, C2AH8, C3AH6, C4AH13), aluminum hydroxide (AH3), C-S-H or portlandite (CH) can be generated. See Table 3, where the relative amounts of the different hydrates in the assemblage are indicated by small “x” and large “X”.
Because ettringite is the hydrate phase, which can (1) bind the most water, (2) forms fast, and (3) causes expansion for the purpose of shrinkage compensation (Nagataki and Gomi 1998 [12]), it is the phase of special interest. Its content can be calculated based on the binder composition by thermodynamic modelling (Figure 6), see Lothenbach 2010 [10] for general information on thermodynamic modelling.
4 Thermodynamic modelling of ettringite contents
The two thermodynamic ettringite modellings are very similar. Both show that binary mixtures of PC + CS̅ develop a minimal amount of ettringite of a few percents, only. However, binary mixtures of CAC + CS̅ can be designed as ettringte systems if a critical amount of CS̅ is contained. Note that the ye’elimite phase (C4A3S̅) is located in Figure 6b on the CA-CS̅ side at 75% CA. Below the ye’elimite-C3S line in Figure 6b, there is a white space, indicating that ettringite is not stable due to an underdosage of CS̅, instead monosulfate is stable.
Ettringite amounts in the reddish- and yellow-colored fields of Figure 6 might cause uncontrolled expansions (see fourth ternary mixture in Table 2, and also Maier 2008 [11]) and should be avoided. In practice this is done by adjusting type and amount of calcium sulfate (CS̅ and/or CS̅H0.5) until shrinkage is compensated providing a dimensional stability. In other words, regions in light blue and green contour colors usually represent controllable amounts of ettringite in terms of shrinkage compensation and fast drying properties, to mention two key properties of ettringite-rich fast-setting systems.
5 Concept for formulating fast-setting mortars
How can a product developer proceed when developing fast-setting formulations?
First of all, it must be stated that given by the large variety of binder products and an even larger amount of possible combinations thereof, there are only some thumb rules, which can be followed. Together, these thumb rules provide a rough concept (Figure 7) for the development of fast-setting formulations.
The first decision is often about high strength and/or wet strength. If not exposed to water or when medium strength is enough, for example for indoor applications, traditional calcium sulfate systems (CS̅) still provide premium solutions in terms of ecology, room climate and price.
As soon as high strength or wet strength is a demand, clinkered materials (PC, CAC, CSA) should be considered. Hereby the product developer can follow different paths. Either focusing on singular binder systems, which in general must be accelerated, or designing a mixed binder system, which in many cases must be retarded to control the intrinsic flash-setting behavior.
CSA is commercially available as CSA clinker or CSA cement. The latter is a mixture of CSA clinker with interground CS̅. CSA clinker (without significant amounts of calcium sulfate) can form ettringite as an early hydration product together with CAH10 und AH3, but in the long term ettringite and CAH10 react to form monosulfate and further AH3 (Winnefeld and Lothenbach 2016 [21], Winnefeld and Lothenbach 2021 [22]). Therefore, CSA clinker is, unlike CSA cement, not classified as an ettringite-forming system (Figure 7).
Regarding mixed binders, the technical instruction paper, TKB-Merkblatt 2015 [19] provides a simple nomenclature for fast-setting mixed binder systems: SZ-B and SZ-T. SZ-B stands for binary “Schnellzemente”, fast-setting cementitious systems. SZ-T stands for ternary fast-setting cementitious systems with additional fast-drying properties. The fast-drying property is related to a higher amount of ettringite, causing a significant higher amount of mixing water to be bound chemically. Such formulations are developed for special adhesives for natural stones of large format ceramic tiles as described by Sieksmeier 2016 [17]. However, there are binary mixtures (CAC + CS̅ or CSA + CS̅), which can have fast-drying properties such as ternary mixtures (CAC > PC + CS̅ and CSA >PC + CS̅; see Table 3 and Figure 7).
Another important field of fast-setting applications are self-leveling flooring mortars such as self-leveling compounds (SLC), self-leveling underlayments (SLU), self-leveling overlayments (SLO) or self-leveling screeds (SLS). Because these mortars are not covered or coated during curing, there is a risk of early drying of the surface layer, resulting in a reduced surface hardness. Especially under harsh conditions on the construction site, like warm temperatures and wind, this risk is high. Regarding SLC or SLU based on ternary ettringite systems, Kighelmann et al. 2008 [6] have shown that a higher amount of ettringite can significantly counteract the risk of a reduced surface hardness. However, such approaches are favorable for indoor applications, because ettringite systems are known to have a limited stability, which may reduce outdoor durability (Lamberet 2005 [8], Sieksmeier 2016 [17]).
Acknowledgements
We acknowledge Alain Zurbriggen (Lucerne/Switzerland) for designing the tetraplots with cadwork v26.




![5 C-CA-CS̅-S0.5 tetraplot with highlighted ternary system planes. (CSAB clinker contain the calcium-sulfoaluminte phase ye’elimite and belite, BCT clinker contain belite, ye’elimite and ternesite, Scholten 2017 [16])](/uploads/images/2022/w300_h200_x600_y438_Materials_Zurbriggen_Fast-setting_mortars_Figure_5-552b63f212a6f2d7.jpeg)
![7 Flow chart of formulation principles for fast-setting mortars. Note that with CS̅ all sorts of calcium sulfates are included, such as α- and β-hemihydrate (CS̅H0.5) and/or anhydrite (CS̅). SZ-B and SZ-T are nomenclatures from TKB (2015) [19] for binary (B) and ternary (T) fast-setting cementitious screeds (SZ for “Schnellzemente”)](/uploads/images/2022/w300_h200_x600_y432_Materials_Zurbriggen_Fast-setting_mortars_Figure_7-9c3d6cbc068a2488.jpeg)
![Table 3 Fast-setting mortar systems in different applications. Ettringite systems are marked with a large red “X”. Data compiled from Lamberet 2005 [8], Pelletier et al. 2010 [13], Winnefeld and Lothenbach 2010 [20], Pelletier et al. 2011 [14], Le Saoût et al. 2013 [9], Chaunsali and Mondal 2015 [3], Winnefeld and Lothenbach 2016 [21], Bullerjahn 2018 [2], Torrens-Martín et al. 2021 [18]](/uploads/images/2022/w300_h200_x600_y407_Materials_Zurbriggen_Fast-setting_mortars_Table_3_NEU-5176448a333aacb2.jpeg)