Research on the effect of CO2/O2 atmosphere on calcined cement clinker
Oxygen-enriched combustion technology has gained considerable attention in the field of combustion due to its ability to reduce and capture CO2. It has potential applications as a carbon capture technology in cement combustion. However, modifying the calcination atmosphere from N2/O2 to CO2/O2 will also bring about changes in coal powder combustion, calcium carbonate decomposition, and clinker firing processes in cement production. Therefore, it is necessary to investigate the relevant influential factors. In this paper, the effects of sintering atmosphere on clinker mineral composition and structure, as well as the size of alite crystals and the hydration microscopic morphology, were analyzed by X-ray diffraction (XRD), Scanning electron microscope (SEM), Differential thermal analysis & Thermogravimetric Analysis (DTA-TG) and Polarized microscope. The results demonstrate that the samples obtained under the CO2/O2 atmosphere exhibit good burnability, and the carbonate decomposition temperature of the samples in air increases by nearly 200 °C compared to that in N2/O2. According to the Rietveld quantitative analysis, a CO2/O2 ratio of 0.59/0.41 represents the most favorable calcination atmosphere for the growth of C3S in cement clinker. When the calcination atmosphere changes from air to CO2/O2, the crystal sizes of alite and belite increase, and the boundaries become more distinct. This research provides significant theoretical support for carbon capture in cement combustion and the optimization of oxygen-enriched combustion technology in cement production.
1 Introduction
With the continuous development of industrial production, global carbon emissions (CO2) have increased from 5 billion t to 22 billion t [1]. In contrast, artificial CO2 utilization remains below 1 billion t. Carbon capture, utilization, and storage (CCUS) technology have been highlighted as the most relevant technology to achieving reduction targets of CO2 emissions, garnering widespread attention [2]. Cement production stands as one of the world’s most energy- and CO2-intensive industrial processes, contributing approximately 7-10% of current global anthropogenic CO2 emissions. The production of cement involves calcination of the raw material limestone and the combustion of fuel, resulting in the generation of a significant amount of CO2. Consequently, this industry is under tremendous pressure to find technological solutions for reducing and capturing CO2 emissions [3]. Oxy-fuel combustion with flue gas recirculation emerges as one of the most promising pathways for decarbonization [4, 5].
Oxy-fuel combustion with flue gas recirculation involves the recirculating of flue gas to the rotary kiln or decomposition furnace while supplying pure oxygen to ensure proper fuel combustion. In this process, high-concentration O2 and CO2 is used instead of air to react with pulverized coal for combustion, resulting in improved combustion efficiency and reduced generation of thermally generated nitrogen oxides. Additionally, this technology enriches CO2 in the flue gas, facilitating easier CO2 capture [6, 7], and is therefore referred to as a carbon capture technology during combustion. However, the transition from N2/O2 to CO2/O2 as the calcination atmosphere in cement production brings about changes in coal combustion, calcium carbonate decomposition, and the clinker firing process. As a result, further investigation is required to identify the relevant influencing factors.
In recent years, researchers have conducted investigations on oxy-fuel combustion with flue gas recirculation technology. Shi Ping [8] conducted simulations to analyze the mild combustion characteristics of propane in different atmospheres. Their findings indicated that CO2 in the auxiliary gas better maintains temperature uniformity in the furnace compared to N2. Further research determined that the most significant reduction in CO emissions occurs when the CO2 concentration is 79%. Xie [9] simulated the combustion characteristics of a novel oxygen-enriched burner that utilizes inert gas to isolate fuel and oxygen, with the aim of reducing NOx content in combustion products. They found that the use of this new burner improved the temperature distribution uniformity and reduced the lowest NOx emission to 1/8 of the original level. Additionally, Lin Tao [10] conducted numerical simulations and experimental research to analyze the effects of oxy-fuel combustion parameters and converter gas conditions on the CO2 concentration and NOx generation in flue gas. They designed a low-energy, high-efficiency CO2 capture method that can reduce the cost of CO2 capture by 30%. Carrasco-Maldonado[11], Koring [12] and the European Cement Research Academy (ECRA) have briefly explained the effect of oxy-combustion on the compressive strength of produced cement in their technical reports. Both studies concluded that the impact is relatively minor. Wang [13] simulated the coal powder combustion in a cement kiln and demonstrated that the implementation of oxy-fuel combustion with flue gas recirculation technology effectively reduces NOx emissions and enhances fuel combustion efficiency. Furthermore, Park [14] performed a numerical simulation to investigate the influence of enriched oxygen on the fine structure and NOx generation characteristics of turbulent syngas non-premixed flames. The results revealed that as the oxygen content increases, the temperature of the oxygen-enriched flame rises, leading to a significant increase in the peak value of NO. In summary, extensive research has been conducted on the effect of oxygen-enriched fuel combustion and the capture of high CO2 concentration. However, there remains a lack of sufficient research on the impact of the CO2/O2 atmosphere on cement clinker calcination.
The present study investigates the effect of various concentrations of CO2/O2 on the calcination process of cement clinker by utilizing the enriched oxygen combustion flue gas recirculation technology. The phase composition and mineral phase distribution of cement clinker under different CO2/O2 atmospheres were analyzed by XRD, SEM, and Polarized microscope. In addition, strength and f-CaO testing were performed to assess the impact of the CO2 calcination atmosphere on the properties, structure, and composition of cement clinker. The objective of this research is to deepen our understanding of the application of oxy-fuel combustion and provide a theoretical foundation for achieving carbon capture in combustion within the cement industry.
2 Experimental
2.1 Preparation of the samples
The raw materials underwent a drying process and were subsequently ground into powders with a fineness of less than 45 μm. After homogenization, samples were collected and subjected to chemical component analysis, evaluating their loss on ignition (LOI). The chemical components of the raw materials are detailed in Table 1.
The raw materials, including limestone, coal slag, gas ash, gold and iron powder, were dried and ground into powders with a particle size of 80 μm, ensuring a standard sieve residue of less than 10%. After homogenization, the powders were prepared as the cement raw meal. To account for the heat consumption during calcination, fly ash was added to the raw meal powder at a weight percentage of 0.81%. The resulting mixtures were then compressed into green pellets with a diameter of Φ30 × 5 mm, applying a pressure of 15 MPa for 120 s. Finally, the green pellets were dried in a drying oven at 65 °C for 30 min in preparation for calcination.
2.2 Analysis and characterization
The synchronous thermal analyzer (TG-DTA, NETZSCH STA 2500) was used to determine the approximate decomposition temperature of the carbonate for the cement raw material. The heating rate was set at 20 K/min, and 10% of N2 was filled in as a protective gas during the test. The FC-6 rapid determination instrument was used to measure the content of free calcium oxide in the cement clinker. Compressive strengths of the cement clinker were measured at 3 days and 28 days, following the ISO method specified in GB/T 17671-2021. To obtain the XRD spectrum of the clinker sample, the X-ray diffraction (D8 Advance X-ray diffractometer from Bruker, Germany) was used under the following test conditions: an accelerating voltage of 40 kV, an accelerating current of 40 mA, a step size of 0.02, a scan time of 1 s per step, and a scan angle of 10~70 °. The polarizing microscope (Olympus BX-51) was used to observe and analyze the microstructure of the clinker. Additionally, the crystal size and morphology of the hydrated clinker samples were characterized using a field emission scanning electron microscope (SEM, UltraPlus, Zeiss).
3 Results and discussion
3.1 Effect of CO2/O2 atmospheres on the decomposition temperature of cement clinker
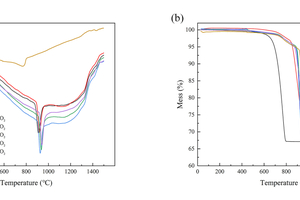
TG-DTA analysis was used to determine the calcination process of cement raw materials in the designed atmosphere combination. The experimental results are shown in Figure 1.
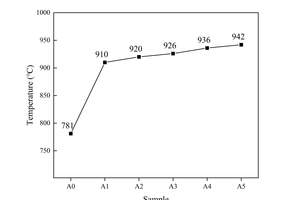
Figure 1 (a) shows a clear endothermic peak was observed for each group of samples (listed in Table 2). The peak temperature was increased with increasing CO2/O2 ratio of the calcination atmosphere by 130 °C, 140° C, 146 °C, 155 °C and 161 °C, respectively. That was a result of the inhibition of the carbonate decomposition process with the increase of the equilibrium partial pressure of CO2 in the atmosphere. Figure 1 (b) shows that with the heating temperature increasing, the weight loss of the samples was observed to be around 35% (listed in Table 2).As the calcination atmosphere changed from air to CO2/O2, the firing process of the clinker has been changed accordingly.
The decomposition reactions of CaCO3 in the CO2/O2 atmosphere primarily occurred within the temperature range of 800-1000 °C, whereas in the air atmosphere, the reactions were concentrated within the range of 600-800 °C. To achieve the desired calcination conditions for the raw materials, the silicon-molybdenum resistance furnace needed to be preheated and heated to approach the peak temperature (as listed in Table 2). Once the desired atmosphere was established by introducing gas into the furnace, the raw material cake was fed into the furnace. Finally, the silicon–molybdenum resistance furnace was heated to 1450 °C and maintained at this temperature for 75 min (based on the typical calcination temperature of cement clinker), followed by an immediate cooling down to room temperature. The specific calcination atmosphere conditions and corresponding sample numbers for this study are presented in Table 2.
3.2 Effect of CO2/O2 atmospheres on the performance of cement clinker
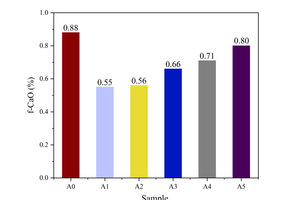
The properties of cement clinker can be negatively influenced by factors such as the presence of a small amount of CaO in a free state, known as free calcium oxide (f-CaO). The content of f-CaO directly indicates the quality of clinker calcination. The existence of f-CaO affects the stability and various properties of cement to varying degrees, making it a crucial factor in production quality control. In order to investigate the influence of the calcination atmosphere on the performance of cement clinker, the free calcium contents of cement clinker samples subjected to different atmospheres were measured. The results are shown in Figure 2.
On the one hand, the f-CaO content of the samples calcined under the air atmosphere was 0.88%, which decreased in all samples calcined under the CO2/O2 atmosphere. On the other hand, as the CO2/O2 ratio increased from 0.39/0.61 to 0.79/0.21, the f-CaO content in the clinker exhibited a significant upward trend. This can be attributed to the addition of a certain concentration of CO2, which alters the equilibrium partial pressure of CO2 in the system and slows down the carbonate decomposition process. Simultaneously, an increase in the proportion of O2 facilitates the absorption of f-CaO by C2S, resulting in a decrease in f-CaO content in clinker with increasing O2 concentration and an increase with increasing CO2 concentration. Furthermore, the f-CaO content of all samples consistently remained below 1.00%, indicating favorable burnability for all samples.
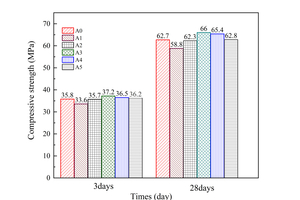
Strength is the primary parameter for evaluating cement products. Figure 3 shows the compressive strength of each cement clinker sample at 3 and 28 days. It can be observed that as the CO2/O2 ratio increases, the strength of the mortar initially decreases and then gradually increases until reaching a CO2/O2 ratio of 0.59/0.41. However, further increase in the CO2/O2 ratio result in a decline in strength. Notably, the highest strength is observed in samples with a CO2/O2 ratio of 0.59/0.41 (sample A3), while sample A1 exhibits the lowest strength among the five samples.
Additionally, it can be observed that sample A4 exhibited the highest strength growth (79.10%) during the 3-28 days age interval (Figure 3). Similarly, sample A3 demonstrated a significant strength growth rate (77.32%). Sample A0, A1 and A2 displayed a similar growth rate of 75.10%, while sample A5 showed a comparatively low strength growth rate. These findings indicate that the calcination atmospheres had an impact on the strength growth of the clinker, and the CO2/O2 ratio of 0.59/0.41and 0.69/0.31 yielded the highest strength growth for the cement clinker. This result indicated that a moderate increase in the CO2/O2 concentration within the experimented CO2/O2 ratio range is beneficial for improving the compressive strength of cement clinker.
3.3 Effect of CO2/O2 atmospheres on the mineral composition of cement clinker
Alite Ca3SiO5 (C3S), belite Ca2SiO4 (C2S), the aluminate phase Ca3Al2O6 (C3A), and the ferrite phase Ca4(Al, Fe)4O10 (C4AF) are the four major phases found in the cement clinker [15, 16]. It is well-known that the crystal structure and mineral composition of cement clinker can undergo significant changes under different calcination regimes [17]. Therefore, to investigate the effect of the calcination atmosphere on the mineral composition of cement clinker, it is necessary to measure the precise amounts of each phase in the clinkers. Quantitative phase analysis (QPA) was performed on six samples that were calcined under different atmospheres using the MAUD software for Rietveld quantitative analysis (Rwp). [18, 19]. Rietveld full-spectrum fitting analysis is a quantitative method that employs the least-squares approach. It incorporates sample characteristics such as crystal structure and diffractive optical factors into the model for calculation, enabling the determination of the mass fraction of each phase [20]. The refinement process involved adjusting the background and scaling parameters, the unit cell parameters, the micro-strain parameters, the atomic position parameters, and the preferred orientation. For the refinement in MAUD, the import phases used were Ca3SiO5 (monoclinic system, Cm (8)), Ca2SiO4 (monoclinic system, P21/n (14)), Ca3Al2O6 (cubic system, Pa-3(205)) and Ca4(Al, Fe)4O10 (orthorhombic system, Ibm2(46)). The experimental patterns were obtained from the XRD machine. Table 3 presents the exact phase compositions refined using MAUD. The weighted profile R value represents the consistency between the calculated and the observed data, and the values obtained (< 12%) indicate the high quality of the Rietveld fits [21-23].
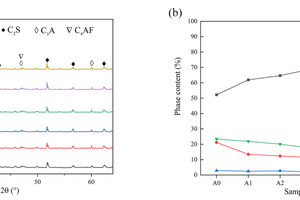
The XRD patterns of clinkers exposed to various CO2/O2 atmospheres are shown in Figure 4 (a). It is evident that the phase species of the clinker prepared under different CO2/O2 atmospheres was similar to that prepared in an air atmosphere. However, the notable distinction lies in the intensity of the characteristic diffraction peaks for each phase. It is important to note that the intensity of diffraction peak cannot represent phase content, as phase content is not discussed in the phase analysis.
The changes in phase content among samples with different calcination atmosphere are depicted in Figure 4 (b). It is observed that the content of C3S and C4AF undergoes significant variations with the alteration of the calcination atmosphere, while the content of C2S and C3A remains relatively stable. Moreover, as the CO2/O2 ratio increases, the content of C3S gradually increases. But after reaching a CO2/O2 ratio of 0.59/0.41, the content of C3S shows a downward trend before increasing again when the CO2/O2 ratio reaches 0.79/0.21.
Based on the results of the TG-DTA analysis in the previous section, it was observed that the change in calcination atmosphere from air to CO2/O2 led to an increase in the decomposition temperature of the raw meal by approximately 150 °C (Figure 5). This increase in temperature can be attributed to two main factors. Firstly, the mineral peak temperatures increased, indicating that the formation of C3S occurred at a higher temperature range. Moreover, the higher decomposition temperature enhanced the efficiency of the raw material decomposition, resulting in an increased content of new ecological CaO with higher activity. This, in turn, favored the synthesis process of C2S and C3S. Secondly, the liquid phase plays a crucial role in the generation of C3S. The higher decomposition temperature of the raw meal increased the amount of liquid phase and reduced the viscosity of the liquid phase. Ultimately, this facilitated the conversion of C2S to C3S.
However, while the increase in decomposition temperature did promote the conversion of C2S to C3S to a certain extent, it was observed that after reaching a CO2/O2 ratio of 0.59/0.41, there was no significant increase in the decomposition temperature of raw materials. Additionally, the change in equilibrium partial pressure within the system occurred as CO2 concentration increased, resulting in the inhibition of carbonate decomposition. Consequently, the production of highly reactive CaO in this temperature range was reduced, hindering the conversion of C2S to C3S and ultimately resulting in a higher content of free calcium in the clinker. This observation is consistent with the variation in f-CaO data discussed in the first section of this chapter. Therefore, the increase in CO2/O2 ratio has both the positive and the negative effects on the content of C3S in the clinker, suggesting the existence of an optimal calcination atmosphere with a CO2/O2 ratio of 0.59/0.41 for achieving the highest C3S content in the clinker. This finding is further supported by the data presented in Table 3, which clearly shows that sample A3 exhibited the highest contents of C3S (69.15%). These findings confirm that the most favorable calcination atmosphere for promoting the growth of C3S in clinker corresponds to a CO2/O2 ratio of 0.59/0.41. The results obtained from these calculations serve as indirect evidence of the variation in cement strength.
3.4 Effect of CO2/O2 atmospheres on the mineral structure of cement clinker
The microstructure of cement plays a vital role in determining its properties. In this experiment, we investigated the influence of the calcination atmosphere on the structure of cement clinker. The petrographic structure of cement clinker was examined using a reflected light microscopy, while the surface morphology of hydrated cement clinker was analyzed using SEM.
3.4.1 Sub-microscopic structural analysis
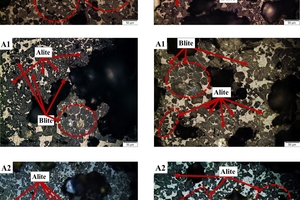
The samples required pretreatment before conducting the petrographic observations. Firstly, the clinker blocks were fixed using phenolic resin, followed by grinding and polishing. To facilitate observations under the reflectance microscope, the surface of the samples was etched with a 1 wt.% ammonium chloride solution. Within the field of view, f-CaO displayed coloration, while alite appeared mainly blue or dark brown in hexagonal shape, and belite appeared as light brown and round structures.
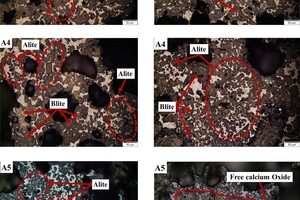
Figure 6 depicts the sub-microstructure of the clinker minerals obtained under different calcination atmospheres. In general, the mineral development in samples exposed to CO2/O2 atmosphere is significantly superior to that in samples exposed to an air atmosphere. Sample A0 displays a low content of alite with irregular shapes, faint edges, and adherence to one another. Within the field of view, belite crystals display distinct morphology and are primarily distributed within the interstices of alite crystals. Sample A1 shows fewer pores but larger size. The alite crystals in this sample appear relatively intact, ranging in size from 30-50 μm, while the boundaries of the larger belite crystals appear more blurred. Within the field of view, the belite crystals are primarily distributed around the alite crystals in the form of mineral nests. The mineralogical characteristics of sample A2 and A4 are similar. The alite crystals are small in size (around 30 μm) and unevenly distributed, with very fuzzy crystal boundaries. Sample A3 exhibits a higher content of alite crystals with a uniform size. Most of the alite crystals in this sample appear as long columns and hexagonal plates, with a few containing inclusions. The belite crystals predominantly exhibit elliptical and egg-shaped forms. In the sample A5, most of the alite crystals range in size between 20-30 µm, with evident mineral aggregation and etching. Free calcium oxide is clearly visible in the form of nests.
Overall, it is evident that the transition from air to CO2/O2 resulted in increased crystal sizes and clearer boundaries for both alite and belite. However, an increase in the CO2 content of the calcination atmosphere led to issues such as larger pore size and incomplete development of the alite crystals, which negatively affects the cement quality. Conversely, decreasing the CO2/O2 ratio resulted in larger crystal size and more defined and complete contours for alite, while belite exhibited smaller crystal sizes with fuzzier contours. Among all the samples, sample A3 exhibited the best performance in terms of crystal morphology.
3.4.2 SEM morphology analysis
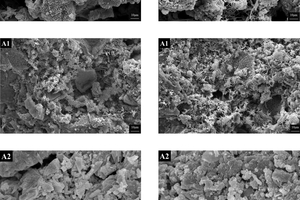
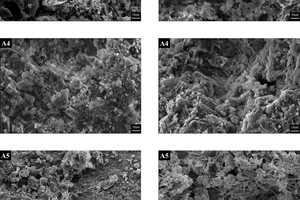
Figure 7 displays the SEM images of the hydrated clinker (3 days) sintered under different calcination atmospheres. For the sample A0, the dominant morphology of the hydration products consisted of hexagonal plate-like crystals of Ca(OH)₂ or hydrated calcium aluminate. However, with a transition from N2/O2 to CO2/O2 in the calcination atmosphere, more needle-like crystals and fibrous C-S-H gels appeared on the hydration particles. These structures interconnected, forming a three-dimensional dense solid structure that was uniformly and densely distributed in sample A1, A3 and A4. Ultimately, these structures became the primary contributors to the strength of the hardened cement. In sample A5, the hydration products were sparsely distributed, with a few long strips of C-S-H gels interspersed among loose spherical particles within the field of view. Sample A2 shows a morphology where slatted gel was present between mineral particles, indicating completed hydration.
In summary, as the calcination environment changed from air to CO2/O2, the surface of the hydrated samples exhibited an increased presence of C-S-H gels and needle-like crystals, which interconnected the dispersed hydration products and formed a three-dimensional dense solid structure. The content and dense distribution of fibrous C-S-H gels and needle-like crystals in the hydration products have a significant impact on the strength of hardened cements. Among all the samples, the morphology of the hydration products in sample A3 and A4 demonstrated the best performance in all samples.
4 Conclusions
Based on this experiment, the following conclusions were drawn regarding the effect of different calcination atmospheres on the properties of cement clinker:
(1) The carbonate decomposition temperature of the samples in air was observed to increase by approximately 200 °C compared to that in CO2/O2 atmosphere. As the CO2/O2 ratio in the calcination atmosphere increased, the endothermic peak temperature of the samples exhibited a corresponding increase of 130 °C, 140 °C, 146 °C, 155 °C, and 161 °C, respectively.
(2) The f-CaO content of all samples consistently remained below 1.00%, indicating their good burnability. The calcination atmospheres with CO2/O2 ratios of 0.59/0.41 and 0.69/0.31 were identified as the optimal conditions for promoting the strength growth of cement clinker. Among these ratios, the CO2/O2 ratio of 0.59/0.41 was found to be the most favorable calcination atmosphere for the growth of C3S in cement clinker, as determined by Rietveld quantitative analysis. Within the range of CO2/O2 ratio examined in this experiment, a moderate increase in CO2 concentration in the calcination atmosphere was observed to enhance the compressive strength of the cement clinker. Notably, the sample with a CO2/O2 ratio of 0.59/0.41 exhibited the highest strength at both 3 days and 28 days, measuring 37.2 MPa and 66.0 MPa, respectively.
(3) The crystal sizes of alite and belite increased, and the boundaries became clearer when the calcination atmosphere changed from air to CO2/O2. As the CO2/O2 ratio decreased, the crystal size of alite increased, and the contours appeared more complete, while the crystal size of belite decreased with more fuzzy contours. In this experiment, the sample with a CO2/O2 ratio of 0.59/0.41 showed the best the crystal morphology among all the samples.
(4) As the calcination environment changed from air to CO2/O2, an increased presence of C-S-H gels and needle-like crystals was observed on the surface of the hydrated samples. Among all the samples, those with CO2/O2 ratios of 0.59/0.41 and 0.69/0.31 exhibited the most favorable sub-microscopic morphology of hydration products and mineral crystals.
Funding
This research was funded by CBMI Construction Co., Ltd. under grant number CBMI-EN-220302-1.
This article by project “Basic theory research and CFD optimization design of oxyfuel combustion in cement kilns” support.